
Michael Trenary
Professor
Contact
Address:
5407A SES, MC 111
Office Phone:
Email:
Related Sites:
About
Our group conducts fundamental studies of the mechanisms of chemical reactions that take place on the surfaces of transition metals. These studies are motivated by a desire to gain a more detailed understanding of the surface chemical reactions that occur in heterogeneous catalysis. Spectroscopic methods are used to identify important surface intermediates and to measure the kinetics of surface reactions. The technique of reflection absorption infrared spectroscopy (RAIRS) has proven to be particularly effective in identifying molecules on surfaces and in distinguishing between adsorbates with subtle differences in structure. We have worked to steadily improve the experimental capabilities of RAIRS and to understand various physical phenomena that influence the spectra. With its high resolution, RAIRS is exquisitely sensitive to different surface chemical species and by recording spectra as a function of time the rates at which reactants are transformed into products can be measured. Such spectroscopic and kinetic studies provide new insights into the mechanisms of surface chemical reactions. For example, by using RAIRS to measure the kinetics of NH formation from atomic nitrogen and hydrogen on the Ru(0001) surface, we were able to establish that quantum mechanical tunneling plays an important role in this reaction. The hydrogenation of nitrogen is a key step in the ammonia synthesis reaction, one of the most industrially significant examples of heterogeneous catalysis.
Body
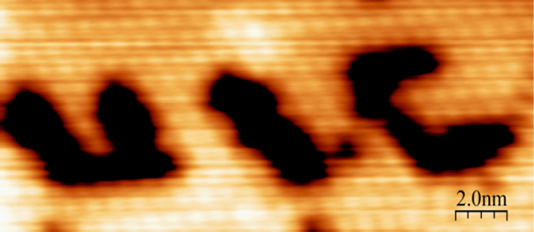
Our spectroscopic studies are often combined with studies of the structure of surfaces with the technique of scanning tunneling microscopy (STM), which can provide atomically-resolved images of surfaces. In addition, through an international collaboration with a Japanese group, we use low temperature STM to study surface chemistry at the single-molecule level. In this collaboration, we observe the properties of individual molecules as they undergo reactions that we have studied at the monolayer level in our UIC laboratories with techniques such as RAIRS. As one example of this approach, we have established the structure of atomic nitrogen on a Pt(111) surface and observed its hydrogenation to NH molecules with both RAIRS and LT-STM. Once an NH-covered Pt(111) surface is formed, the STM tip can then be used to selectively dissociate individual NH molecules, allowing letters to be written on surfaces at the atomic-scale.
Selected Publications
1. David L. Molina, Mark Muir, and Michael Trenary, “The Influence of Palladium on the Hydrogenation of Acetylene on Ag(111)”, J. Chem. Phys 154, 184701 (2021). DOI: 10.1063/5.0050587
2. Dominic Esan and Michael Trenary, “Interaction of CO with Pt Nanoclusters on a Graphene-Covered Ru(0001) Surface”, J. Chem. Phys., 154, 114701 (2021). DOI: 10.1063/5.0042686
3. Mark Muir, David L. Molina, Arephin Islam, Mohammed Abdel-Rahman and Michael Trenary, “Adsorption Properties of Acrolein, Propanal, 2-Propenol, and 1-Propanol on Ag(111)”, Phys. Chem. Chem. Phys., 22, 25011-25020, (2020). DOI: 10.1039/D0CP04634
4. Mark Muir, David L. Molina, Arephin Islam, Mohammed K. Abdel-Rahman, and Michael Trenary, “Selective Hydrogenation of Acrolein on a Pd/Ag(111) Single-Atom Alloy Surface”, J. Phys. Chem. C 124, 24271-24278 (2020), DOI:10.1021/acs.jpcc.0c08094
5. Mohammed Abdel-Rahman and Michael Trenary, “Propyne hydrogenation over a Pd/Cu(111) single-atom alloy studied with ambient pressure infrared spectroscopy”, ACS Catalysis 10, 9716-9724 (2020) DOI: 10.1021/acscatal.0c02475
6. Mark Muir and Michael Trenary, “Adsorption of CO to Characterize the Structure of a Pd/Ag(111) Single-Atom Alloy Surface”, J. Phys. Chem. C 124, 14722-14729 (2020) DOI: 10.1021/acs.jpcc.0c0426
7. Emiko Kazuma, Minhui Lee, Jaehoon Jung, Michael Trenary, Yousoo Kim, “Single-Molecule Study of a Plasmon-Induced Reaction of a Strongly Chemisorbed Molecule”, Angew. Chem. Int. Ed. 59, 7960–7966 (2020) DOI: 10.1002/anie.202001863.
8. Emiko Kazuma, Jaehoon Jung, Hiromu Ueba, Michael Trenary, and Yousoo Kim, “Real-space and real-time observation of plasmon-induced chemical reaction of a single molecule”, Science, 360, 521-526 (2018). DOI: 10.1126/science.aao0872
Notable Honors
2011, Fellow, American Chemical Society
2009, Fellow, American Association for the Advancement of Science
2008, Teaching Recognition Award, University of Illinois at Chicago
2004, Special Creativity Award, National Science Foundation
2002, Fellow, American Vacuum Society
1990, University Scholar Award, University of Illinois
1989, Teacher-Scholar Award, Camille and Henry Dreyfus Foundation
Education
B.S. in Chemistry, University of California at Berkeley, 1978.
Ph.D. in Physical Chemistry, Massachusetts Institute of Technology, 1982.
Postdoctoral Fellow, University of Pittsburgh, 1982-1984.
Professional Memberships
- Japanese Society for the Promotion of Science Fellowship, 2010
- Eminent Visiting Scientist, RIKEN, Wakoshi, Japan, 2004
- Guest Professor, Tohoku University, Sendai, Japan, 1998
- Visiting Scholar, Waseda University, Tokyo, Japan, 1994-1995