
Tim Keiderling
Emeritus Professor
Contact
Address:
5407B SES, MC 111
Office Phone:
Email:
Related Sites:
About Heading link
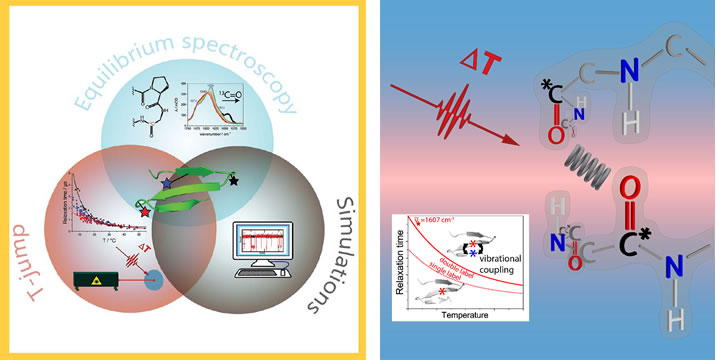
Our research is primarily focused on the use of spectroscopic techniques to determine biomolecular structure and follow its dynamic changes. My group is recognized for developing new IR and vibrational circular dichroism (VCD) based optical spectroscopic methods and applying them to the study of peptide and protein conformation and folding. We have developed ab initio QM methods to model and interpret our peptide IR, Raman and VCD spectra. Our spectral studies probe equilibrium and thermodynamic changes, as well as microsec dynamics using IR and fluorescence detected T-jump methods. Most of these studies address model peptide systems to exemplify fundamental secondary structure types and to model small folding units in proteins. Our studies also use NMR structure determination to generate models for folded structures and MD methods to develop models for folding processes and intermediate structures on the pathway. Recently we have begun studies related to protein and peptide aggregation and fibril formation, processes important in many biomedical conditions.
Additionally, the group previously studied folding of larger proteins, with an emphasis on b-sheet dominant structures, aggregates, and models for protein membrane interaction and folding studies. Perturbation effects on protein and protein complex structures, such as disulfide reduction, surface binding, pH and thermal variation are studied with equilibrium and dynamic (stop-flow) IR, CD and fluorescence methods.
Earlier work allowed us to refine protein structure determination in terms of fractional secondary structure with using various pattern recognition and statistical methods to interpret optical spectra, which can correlate with NMR studies, H-D exchange, as well as kinetically based fluorescence and electronic CD spectral measurements. All of these are useful for dynamic study of structural changes such as occur during the protein folding process.
Body Heading link
.
Selected Publications
1. “Inter-residue Coupling and Equlibrium Unfolding of PPII Helical peptides. Vibrational spectra enhanced with 13C isotopic labeling,” Heng Chi, Ahmed Lakhani, Anjan Roy, Marcelo Nakaema, Timothy A. Keiderling, Journal of Physical Chemistry B, 114,12744-12753 (2010) DOI: 10.1021/jp106095q
2. “Infrared, VCD and Raman Spectral Simulations for b-Sheet Structures with Various Isotopic Labels, Interstrand and Stacking Arrangements using Density Functional Theory,” William R. W.Welch, Jan Kubelka, Timothy A. Keiderling, Journal of Physical Chemistry B, 117, 10343−10358 (2013) DOI: 10.1021/jp4056126
3. “Insight into the Packing Pattern of β2 Fibrils. A Model Study of Glutamic Acid Rich Oligomers with 13C Isotopic Edited Vibrational Spectroscopy,” Heng Chi, William R. W. Welch, Jan Kubleka, Timothy A. Keiderling, BioMacromolecules, 14, 3880−3891 (2013) DOI: 10.1021/bm401015f
4. “Dimethyl Sulfoxide Induced Destabilization and Disassembly of Various Structural Variants of Insulin Fibrils Monitored by Vibrational Circular Dichroism,” Ge Zhang, Viktoria Babenko, Wojciech Dzwolak and Timothy A. Keiderling, Biochemistry, 54, 7193−7202 (2015) DOI:10.1021/acs.biochem.5b00809
5. “Sensing site‐specific structural characteristics and chirality using vibrational circular dichroism of isotope labeled peptides,” Timothy A. Keiderling, Chirality, 29, 763–773 (2017) DOI: 10.1002/chir.22749
6. “Theory of Molecular Vibrational Zeeman Effects as Measured with Circular Dichroism,” Timothy A. Keiderling and Petr Bouř, Physical Review Letters, 121, 073201 (2018) DOI: 10.1103/PhysRevLett.121.073201
7. “Isotopically Site-Selected Dynamics of a Three-Stranded b-Sheet Peptide Detected with Temperature-Jump IR-Spectroscopy,” David Scheerer, Heng Chi, Dan McElheny, Timothy A. Keiderling, Karin Hauser, Journal of Physical Chemistry, 122, 10445-10454 (2018) http://dx.doi.org/10.1021/acs.jpcb.8b08336, Supplementary Cover Paper
8. “Enhanced sensitivity to local dynamics in peptides by use of temperature-jump IR-spectroscopy and isotope labeling,” Scheerer, David; Chi, Heng; McElheny, Dan; Keiderling, Timothy; Hauser, Karin, Chemistry a European Journal, 26, (on line, 2020) https://doi.org/10.1002/chem.201904497
9. “Structure of condensed phase peptides : Insights from VCD / ROA techniques,” Timothy A. Keiderling, Chemical Reviews (on line , 2020) http://dx.doi.org/10.1021/acs.chemrev.9b00636.
Education
BS, Loras College, 1969
PhD, Princeton University, 1974
Postdoctoral Fellow, University of Southern California,1973-76
Fulbright Senior Research Fellow, Max Planck Institute for Quantum Optics, Munich, 1984
University of Illinois Scholar, 1991-1994
Senior Visitor, Oxford Centre for Molecular Sciences, Oxford University, 1994
Guggenheim Fellow and Guest Professor, University of Freiburg, Germany, 2004-05, University of Padova, Italy, 2005
Humboldt Research Award and Guest Professor, University of Konstanz, Germany, 2011-12 and 2017-18