
Wonhwa Cho
Liberal Arts and Sciences Science Endowed Chair and UIC Distinguished Professor and Head
Contact
Address:
4508 SES, MC 111
Office Phone:
Email:
Related Sites:
About Heading link
Chemical biology and drug discovery: Molecular sensors for quantitative optical imaging of cellular processes: Lipid-targeting drugs for cancer and neurodegenerative diseases: Roles of membrane lipids and lipid binding proteins in cancer, aging, and neurodegenerative diseases
Cellular processes, such as cell signaling, are mediated by myriad of molecular interactions that are exquisitely coordinated and tightly regulated. Our recent studies have shown that membrane lipids play key roles in orchestrating this complex regulation. Our current research focuses on i) understanding how lipids perform their regulatory actions, ii) discovering their new functions in health and disease, iii) and developing new and novel lipid-targeting drugs to treat diverse human diseases caused by dysfunctional lipid-mediated processes, including cancer, neurodegenerative diseases, and autoimmune diseases diseases.
Body Heading link
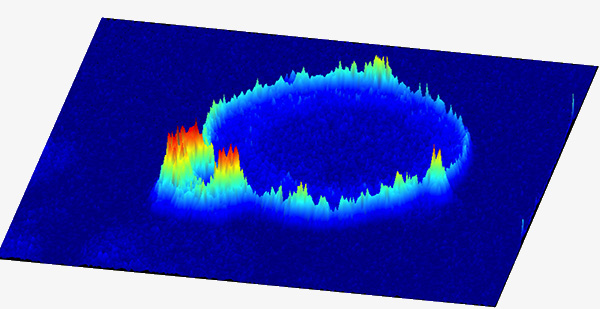
(1) Seeing is believing and it is numbers that matter
Lipids are dynamic regulatory molecules and their cellular function depends on their subcellular location and local concentration. Our innovative in situ quantitative lipid imaging technology (Nature Chemistry, 2011; Nature Chemical Biology, 2017; Molecular Cell, 2018) has not only demonstrated high-resolution spatiotemporal dynamics of various lipids but also helped us discover their new cellular function in health and disease. We continue to develop fluorescence-based ratiometric sensors for simultaneous and quantitative imaging of lipids, lipid-binding proteins, and membrane dynamics in diverse live cells and tissues, including patient-derived samples, to elucidate the complex mechanisms of lipid-mediated cell regulation and identify new targets for drug discovery. We are also developing new chemical biology tools that allow spatiotemporally specific manipulation of lipid actions in live cells.
Body2 Heading link
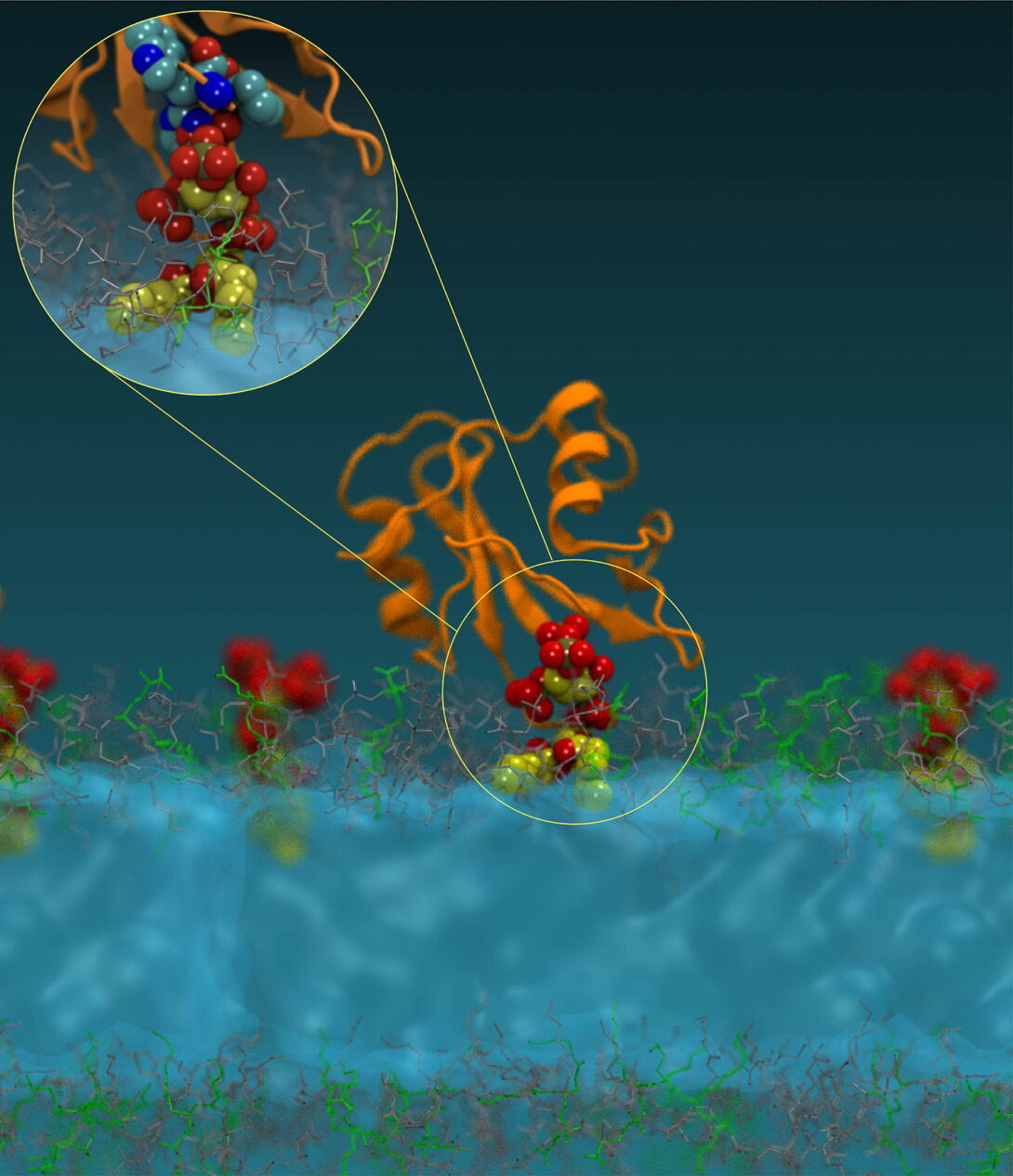
(2) Lipids as master regulators of cellular processes and new targets for drug discovery
Our studies have shown that membrane lipids serve as tunable switches that spatiotemporally orchestrate compartmentalization, mutual interaction, and activation of cellular proteins including those involved in human diseases (Molecular Cell, 2012: Molecular Cell, 2016). Our work has also shown that the cellular activity of these proteins can be specifically controlled by modulating their lipid binding. Based on these discoveries, we have developed a first-in-class small molecule lipid-protein interaction (LPI) inhibitor for a non-receptor tyrosine kinase (Nature Chemical Biology, 2023). LPI inhibitors offer major advantages over conventional ATP-competitive inhibitors in terms of specificity and refractoriness to drug resistance. Our current work focuses on developing novel LPI inhibitors as drug candidates for cancer, neurodegenerative diseases, and autoimmune diseases diseases.
Body3 Heading link
(3) Multi-talented cholesterol: An emerging star in cancer and neurodegenerative diseases
Cholesterol is a major lipid component of the mammalian plasma membrane and its involvement in cardiovascular diseases is well documented. More recently, cholesterol has been linked to human cancers and neurodegenerative diseases, most notably Alzheimer’s disease, but the underlying mechanisms are poorly understood. We recently discovered that cholesterol directly and specifically activates cell signaling proteins, which leads to cell growth and proliferation (Nature Communications, 2012, 2014). Using our ratiometric cholesterol sensors and site-specific cholesterol modulation system, we also discovered that spatiotemporally specific enrichment of cholesterol is crucial for its cellular regulatory function (Nature Chemical Biology, 2017). Furthermore, cancer cells maintain cell type-specific local cholesterol levels that drive cancer progression and also serve as new cancer biomarkers. Based on these exciting new discoveries, we are currently developing new cholesterol-targeting LPI inhibitors for various human cancers, including breast cancer and colorectal cancer. The same approach is also being applied to the development of novel therapy for Alzheimer’s disease and autoimmune diseases.
Selected Publications
1. Yoon, Y., Lee, P.J., Kurilova, S., and Cho, W. (2011), In Situ Quantitative Imaging of Cellular Lipids Using Molecular Sensors. Nature Chemistry 3, 868-74. [Featured in ASBMB Today, RSC Chemistry World, ScienceDaily and UIC News]
2. Chen, Y., Sheng, R., Kallberg, M., Silkov, A., Tun, M.P., Bhardwaj, N., Kurilova, S., Hall, R.A., Honig, B., Lu, H., and Cho, W. (2012) Genome-Wide Functional Annotation of Dual Specificity Protein- and Lipid-Binding Modules That Regulate Protein Interactions. Molecular Cell 46, 226-237. [Featured in Faculty of 1000, ScienceDaily and UIC News]
3. Sheng, R., Chen, Y., Gee, H. Y., Stec, E., Melowic, H.R., Blatner, N.R., Tun, M.P, Kim, Y., Kallberg, M., Fujiwara, T., Hong, J.H., Kim, K.P., Lu, H., Kusumi, A., Lee, M.G. and Cho W. (2012) Cholesterol Modulates Cell Signaling and Protein Networking by Specifically Interacting with PDZ Domain-Containing Scaffolding Proteins. Nature Communications 3, 1249 DOI: 10.1038/ncomms2221. [Featured in National Institute of General Medical Sciences News and UIC News]
4. Sheng, R., Kim, H., Lee, H., Xin, Y., Chen, Y., Tian, W., Choi, J.-C., Doh, J., Han, J.-K. and Cho, W. (2014), Cholesterol Selectively Activates Canonical Wnt Signaling over Non-Canonical Wnt Signaling. Nature Communications 5:4393 doi: 10.1038/ncomms5393
5. Park, M-J, Sheng, R., Silkov, A., Jung, D-J, Wang, Z-G, Xin, Y., Kim, H., Thiagarajan-Rosenkranz, P., Song, S., Yoon, Y., Nam, W., Kim, I., Kim, E., Lee, D-G, Chen, Y., Singaram, I., Wang. L. Jang, M.H., Hwang, C.-S., Honig, B., Ryu, S.H., Lorieau, J., Kim, Y-M, and Cho, W. (2016) SH2 domains serve as lipid binding modules for pTyr-signaling proteins Molecular Cell 62, 7-20. [Highlighted in Nature Reviews in Molecular Cell Biology (May, 2016) and Cancer Discovery; Recommended in Faculty of 1000 Prime being of special significance]
6. Liu, S.-L., Sheng, R., Jung, J.H., Wang, L., Stec, E., O’Connor, M.J. Song, S., Bikkavilli, R.K., Baek, K., Winn, R.A., Lee, D., Ueda, K., Levitan, I., Kim, K.-P., and Cho, W. (2017) Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol Nature Chemical Biology, 13, 268-274
7. Liu, S.-L., Wang, Z., Hu, Y., Xin, Y., Gorai, S., Sharma, A., Zhou, X., Gong, L-W., Hay, N., Zhang, J. and Cho, W. Quantitative lipid imaging reveals a new signaling function of phosphatidylinositol-3,4-bisphosphate: Isoform and subcellular site-specific activation of Akt (2018) Molecular Cell 71, 1092-1104 [Featured in Preview]
8. Zhang, Y., Bulkley, D.P., Xin, Y., Roberts, K.J., Asarnow, D.E., Sharma, A., Myers, B.R., Cho, W.*, Cheng, Y.*, Beachy*, P.A. Structural basis for cholesterol transport-like activity of the Hedgehog receptor Patched (2018) Cell 175, 1352-1364 (*Co-corresponding authors)
9. Singaram, I., Sharma, A., Pant, S., Lihan, M., Park, M.-J., Pergande, M., Buwaneka, P., Hu, Y. Mahmud, N. Kim, Y.-M. Cologna, S. Gevorgyan, V. Khan, I. Tajkhorshid, E. and Cho, W. Targeting lipid-protein interaction to treat Syk-mediated acute myeloid leukemia (2023) Nature Chemical Biology 19, 230-250 [Highlighted in Cancer Discovery]
Service to Community
Editorial Board, Progress in Lipid Research, 2010-present
Head of the Membrane Biological Physics Section, Faculty Opinions (formerly Faculty 1000Prime), 2021-present
Associate Editor, Frontiers in Cell and Developmental Biology, 2022-present
Associate Editor, Frontiers in Molecular Biosciences, 2022-present
Notable Honors
1992, Arthritis Investigator, Arthritis Foundation
1999, Established Investigator, American Heart Association
2009, Graduate Mentoring Award, University of Illinois at Chicago
2015, Researcher of the Year Award, University of Illinois at Chicago
2018, Avanti Award, Biophysical Society
2018, Elected Fellow, American Association for the Advancement of Science
Education
BS, Seoul National University, 1980; MS, Seoul National University, 1982
PhD, University of Chicago, 1988
Postdoctoral Fellow, California Institute of Technology, 1988-1990