UIC researchers report on new class of redox mediators for Li-O2 batteries
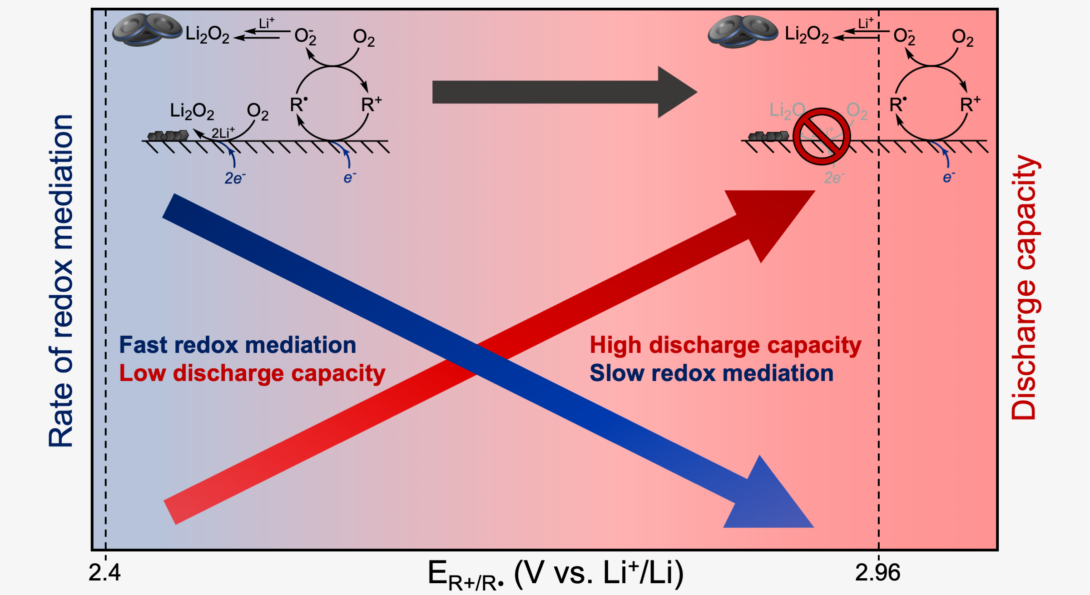
UIC Chemistry researchers recently published a paper in Nature Chemistry on redox mediation for Li-O2 batteries. The work comes from a collaboration between researchers at UIC (Erik Askins, Ksenija Glusac), SLAC National Accelerator Laboratory (Marija Zoric) and Argonne National Laboratory (Matthew Li, Rachid Amine, Larry Curtiss and Khalil Amine). The paper reports a new class of redox mediators (RM) based on a family of triarylmethyl carbocations and their remarkable improvements to the discharge of Li-O2 batteries. These triarylmethyl cations operate through an outer-sphere electron transfer pathway which mitigates deposition of electronically insulating Li2O2 on conductive electrodes. The study also unearthed crucial relationships between RM reduction potential, bimolecular reaction kinetics and battery discharge capacity. Take a look here!