
Ksenija Glusac
Professor
Contact
Address:
5105 SES
Office Phone:
Email:
Related Sites:
About
The Glusac research group is at the forefront of scientific innovation, with a focus on photo- and electro-catalytic processes critical to advancements in solar fuels, CO₂ capture and conversion, and energy storage technologies. Our work integrates cutting-edge techniques in molecular synthesis, electrochemistry, and spectroscopy. We actively welcome collaboration and inquiries as we aim to drive progress in energy research and technology. Our lab conducts three primary lines of investigation:
Molecule-Electrode Hybrid Materials: We explore novel molecule/electrode hybrid materials formed by attaching, either covalently or noncovalently, tunable molecular electrocatalysts to the surface of conductive electrodes. We are particularly interested in understanding how the electrochemical behavior of molecular catalysts changes when they are immobilized onto the electrode surfaces and exposed to the electric fields that develop at the electrode/electrolyte interfaces. The catalysts are selected among those that drive transformations that are important for green energy applications, such as hydrogen evolution reaction, carbon dioxide reduction and oxygen reduction reaction.
Body
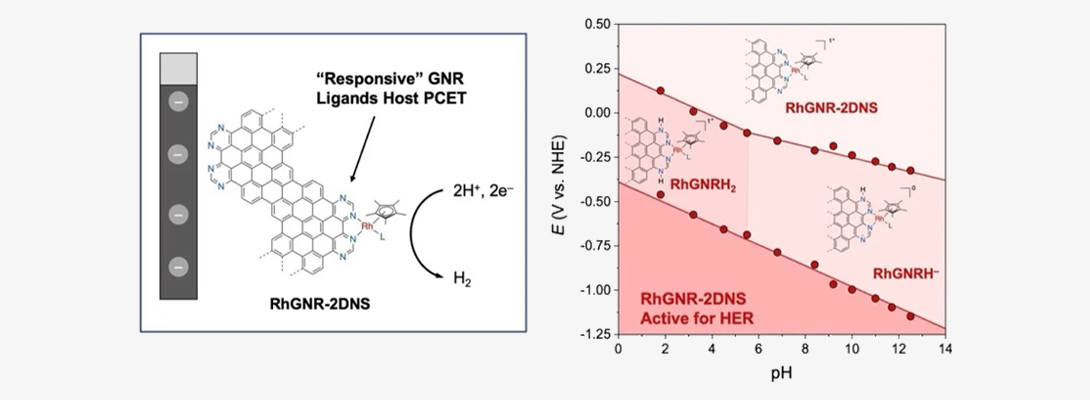
,
Body
Redox Mediation: We investigate molecular redox shuttles that can transport hydride ions, hydrogen atoms and electrons. We investigate factors that control thermodynamics and kinetics of electrochemical or photochemical generation of redox shuttles and the efficiency with which they deliver redox equivalents to the sites where chemical reactions take place. Our approach is in many ways inspired by the ways nature shuttles charge carriers via its quinone, flavin and NADH-based cofactors. We also investigate the applications of our redox shuttles in processes such as electrochemical and photochemical CO2 reduction, as well as Li-oxygen batteries.
Body
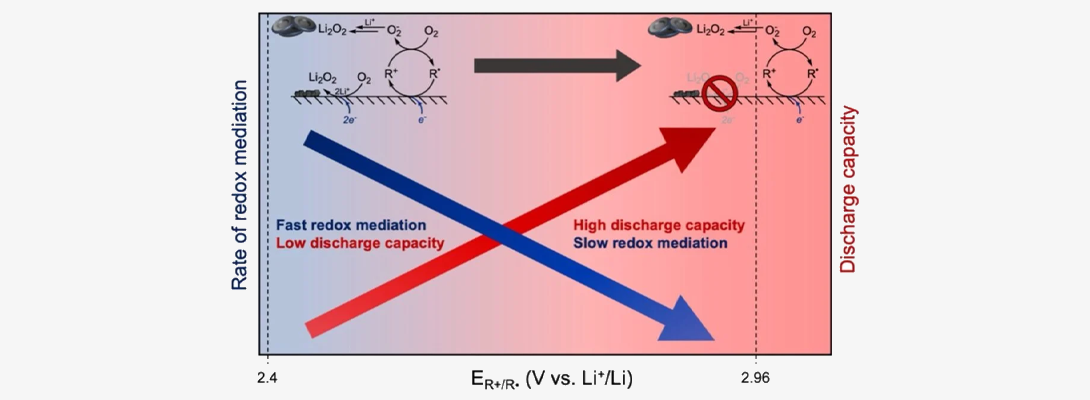
.
Body
Integrated carbon dioxide capture and conversion: In this project, we investigate novel chemical approaches to combining carbon dioxide capture and its direct conversion to value-added chemicals using solar energy. For this purpose, we investigate structures that enable the assembly of CO2-capture sorbents, light-harvesting groups and catalysts into complex “molecular photoreactors” for target transformations.
a
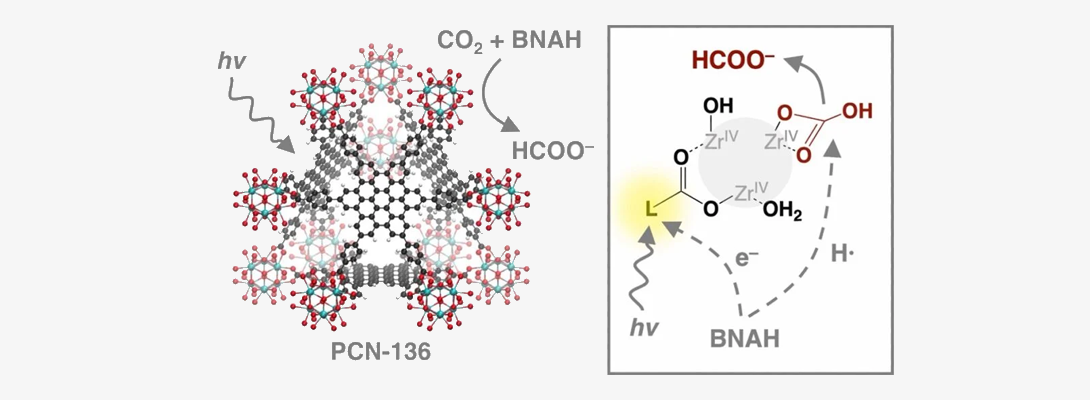
;
Selected Publications
• Askins, E; Sarkar, A; Navabi, P; Kumar, K; Finkelmeyer, S; Presselt, M; Cabana, J; Glusac, K.
Interfacial Electrochemistry of Catalyst-Coordinated Graphene Nanoribbons
J Am Chem Soc 2024, DOI: 10.1021/jacs.4c05250
• Askins, E.; Zoric, M.; Amine, R.; Amine, K.; Glusac, K.
Li-O2 Battery Discharge Redox Mediation by Triarylmethyl Cations
Nat Chem 2023, doi.org/10.1038/s41557-023-01268-0
• Glusac, K. D; Saicic, R. N.,
Are science and technology friends or foes?
Nat Chem 2023, https://doi.org/10.1038/s41557-023-01171-8
• Ilic, S.; Gesiorski J. L.; Weerasooriya R. B. and Glusac, K. D.
Biomimetic Metal-Free Hydride Donor Catalysts for CO2 Reduction
Acc Chem Res 2022, https://doi.org/10.1021/acs.accounts.1c00708
• Askins, E. J.; Zoric, M. R.; Li, M.; Luo, Z.; Amine, K. and Glusac, K. D.
Toward a Mechanistic Understanding of Electrocatalytic Nanocarbon
Nat Commun 2021, https://doi.org/10.1038/s41467-021-23486-1
• C.-H. Lim, S. Ilic, A. Alherz, B. T. Worrell, S. S. Bacon, J. T. Hynes, K. D. Glusac. and C. B. Musgrave
Benzimidazoles as Metal-Free and Recyclable Hydrides for CO2 Reduction to Formate
J. Am. Chem. Soc, 2019, DOI: 10.1021/jacs.8b09653.
• Ilic, U. P. Kadel, Y. Basdogan, J. A. Keith and K. D. Glusac
Thermodynamic Hydricities of Biomimetic Organic Hydride Donors
J. Am. Chem. Soc., 2018, DOI: 10.1021/jacs.7b13526.
• K. D. Glusac,
What Has Light Ever Done for Chemistry?
Nat Chem., 2016, 8, 734-735 DOI:10.1038/nchem.2582.
• D. Zhou, R. Khatmullin, J. Walpitha, N. A. Miller, H. L. Luk, S. Vyas, C. M. Hadad, K. D. Glusac,
The Fast Excited-State Heterolytic C–OH Bond Cleavage of 9-hydroxy-10-methyl-9-phenyl-9,10-dihydroacridine: A Photoemitter of Hydroxide Anions,
J. Am. Chem. Soc, 2012, 134, 11301, https://pubs.acs.org/doi/10.1021/
• E. Mirzakulova, R. Khatmullin, J. Walpita, T. Corrigan, N. M. Vargas-Barbosa, S. Vyas, S. Ottikkal, S. Manzer, C. M. Hadad, K. D. Glusac,
Electrode-assisted Catalytic Water Oxidation by a Flavin Derivative,
Nat Chem., 2012, 4, 794. DOI: 10.1038/nchem.1439.Nat Chem., 2012, 4, 794. DOI: 10.1038/nchem.143
Education
B.S., University of Belgrade, Serbia 1999
Ph.D., University of Florida 2003
ACS PRF Postdoctoral Fellow, Stanford University 2004-2006