Ksenija Glusac
Professor
Contact
Address:
5105 SES
Office Phone:
Email:
Related Sites:
About Heading link
We study conceptually new catalytic approaches for solar fuel-forming reactions. The mainstream research in this area explores transition-metal-based systems to catalyze O2/H2 evolution and CO2 reduction. These catalysts are often made of rare and toxic metals, which disables their wide usage. To provide less expensive and more environmentally friendly alternatives, we aim to discover earth-abundant catalytic motifs that utilize solely organic compounds made of C, H, N and O.
Three projects are currently under investigation in our labs:
Catalyst-grafted Graphene Nanoribbons:
Body Heading link
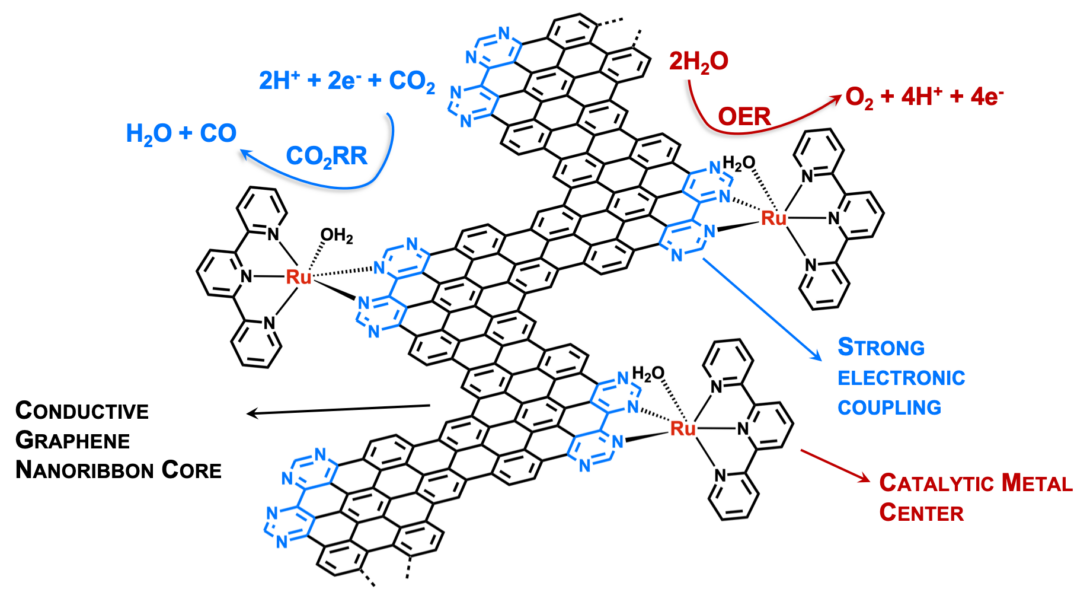
In this project, we investigate electrochemical transformations that are catalyzed by a novel type of catalysts composed of conductive graphene nanoribbon platforms and chemically tunable transition metal centers. We are interested in evaluating the factors that control the charge transport through the carbon network and the effect of large conjugated ligand on the catalytic performance of transition metals. Our fundamental studies aim to identify the motifs for future graphene-based electrode materials for batteries, fuel cells and photoelectrochemical cells. This project is currently funded by NSF.
Photocatalytic CO2 reduction using NAD /NADH analogs.
Body Heading link
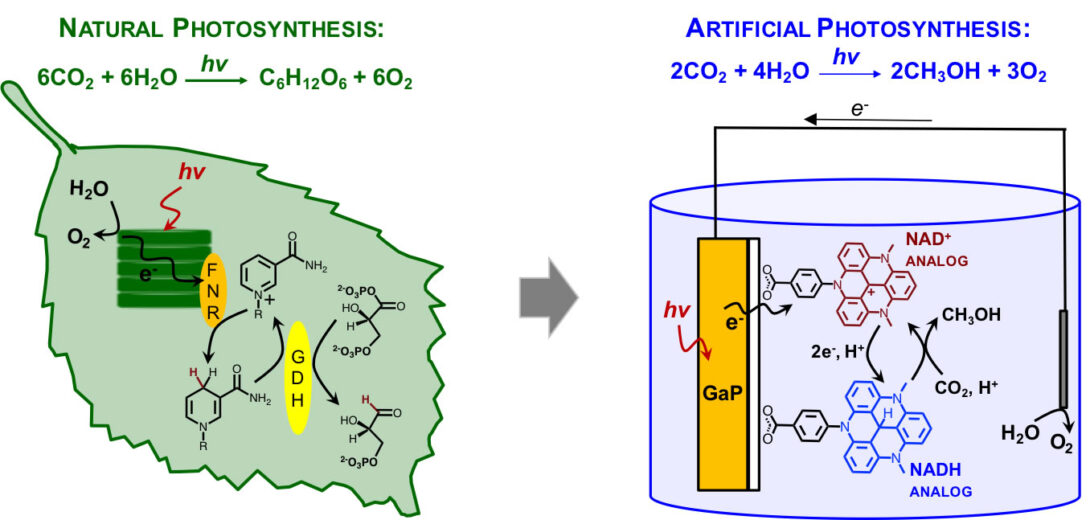
In this biomimetic approach, the CO2 reduction is expected to occur by a sequence of three hydride transfer steps from NADH analogs, which are photo-generated from the corresponding NAD analog dyes (see figure). The advantage of such an approach is that the selectivity for methanol production will be achieved, while the undesired proton reduction will be suppressed by metal-free catalysts. We recently demonstrated the efficient photoreduction of NAD analog dyes on GaP, while our current efforts aim to evaluate the hydricities of model NADH analogs and their reactivity toward CO2 reduction. This study will provide important mechanistic insights into the CO2 reduction catalysis observed by others using simple organic catalysts (such as pyridinium ion). This project is currently funded by ACS PRF.
Light Harvesting By Graphene Quantum Dot Assemblies:
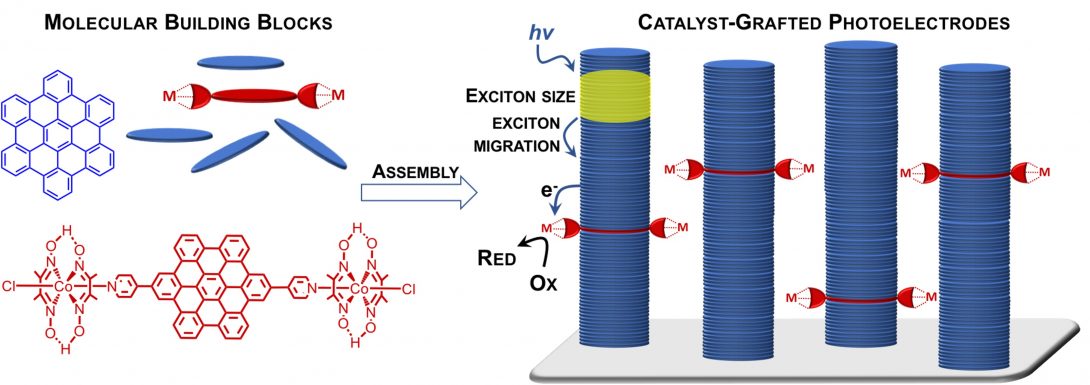
This project involves a study of graphene quantum dot (GQD) assemblies as light harvesting elements, with potential applications for solar fuel production. The absorption frequency can be tuned by varying the GQD size, while the catalyst for solar fuel forming processes can be attached to the edges. The aim of this project is to use ultrafast laser spectroscopy to investigate the factors that control the exciton size and migration in GQDs, as well as rates of photoinduced charge separation at the catalytic sites. This project is currently funded by DOE.
While this research is motivated by the applications in energy storage, our approach is quite fundamental in nature and addresses the key aspects of important chemical processes, such as O-O bond formation and hydride transfer. Our aim is to facilitate the discovery of a new generation of catalysts by providing fundamental mechanistic insights.
Selected Publications
1. V. Singh, M. R. Zoric, G. N. Hargenrader, A. J. S. Valentine, O. Zivojinovic, D. R. Milic, X. Li, and K. D. Glusac, Exciton Coherence Length and Dynamics in Graphene Quantum Dot Assemblies, 2020, J. Phys. Chem. Lett, doi.org/10.1021/acs.jpclett.9b03384.
2. M. Zoric, V. Singh, S. Warren, R. R. Khatmullin, K. D. Glusac, Electron Transfer Kinetics at Graphene Quantum Dot Electrodes, 2019, ACS Appl. Mater. Interfaces, doi.org/10.1021/acsami.9b14161.
3. C.-H. Lim, S. Ilic, A. Alherz, B. T. Worrell, S. S. Bacon, J. T. Hynes, K. D. Glusac. and C. B. Musgrave, “Benzimidazoles as Metal-Free and Recyclable Hydrides for CO2 Reduction to Formate”, J. Am. Chem. Soc, 2019, DOI: 10.1021/jacs.8b09653.
4. Zoric, U. P. Kadel and K. D. Glusac, “Co-catalysis: Role of Organic Cations in Oxygen Evolution Reaction on Oxide Electrodes”, ACS Appl. Mater. Interfaces, 2018, DOI: 10.1021/acsami.8b10232.
5. Ilic, U. P. Kadel, Y. Basdogan, J. A. Keith and K. D. Glusac, “Thermodynamic Hydricities of Biomimetic Organic Hydride Donors”, J. Am. Chem. Soc., 2018, DOI: 10.1021/jacs.7b13526.
6. K. D. Glusac, What Has Light Ever Done for Chemistry?, Nat. Chem., 2016, 8, 734-735 DOI:10.1038/nchem.2582.
7. J. Walpita, Y. Xin, R. Khatmullin, H. L. Luk, C. Hadad, K. D. Glusac, Proton-Coupled Electron Transfer in Weakly Coupled Systems: A Case Study Involving Acridinol and Phenanthridinol Pseudobases, J. Phys. Org. Chem, 2015, DOI: 10.1002/poc.3516.
8. X. Yang, J. Walpita, E. Mirzakulova, S. Vyas, S. F. Manzer, C. M. Hadad, K. D. Glusac, Mechanistic Studies of Electrode-Assisted Catalytic Oxidation by Flavinium and Acridinium Cations, ACS Catal, 2014, 4, 2635-2644. DOI: 10.1021/cs5005135.
9. X. Yang, J. Walpitha, D. Zhou, H. L. Luk, S. Vyas, R. S. Khnayzer, S. Chandra, C. M. Hadad, F. N. Castellano, A. I. Krylov, K. D. Glusac, Toward Organic Photohydrides: Excited-State Behavior of 10-Methyl-9-Phenyl-9.10-dihydroacridine, J. Phys. Chem. B, 2013, 117, 49, 15290. DOI: 10.1021/jp401770e. Part of the Michael D. Fayer Festschrift.
10. E. Mirzakulova, R. Khatmullin, J. Walpita, T. Corrigan, N. M. Vargas-Barbosa, S. Vyas, S. Ottikkal, S. Manzer, C. M. Hadad, K. D. Glusac, Electrode-assisted Catalytic Water Oxidation by a Flavin Derivative, Nat. Chem., 2012, 4, 794. DOI: 10.1038/nchem.1439. This work was highlighted by C&EN News, 2012, Vol. 90, Issue 35, p.10; http://cen.acs.org/articles/90/i35/Organocatalyst-Splits-Water.html
Education
B.A., University of Belgrade, Serbia 1999
Ph.D., University of Florida 2003
ACS PRF Postdoctoral Fellow, Stanford University 2004-2006