
Lawrence Miller
Associate Professor
Contact
Address:
5205 SES, MC 111
Office Phone:
Email:
Related Sites:
About
Understanding biological complexity requires dynamic, spatially resolved data on protein abundance and activity. We develop new technologies for bioimaging and sensing, with a particular emphasis on chemical probes for visualizing, analyzing and screening protein-protein interactions. Much of our work leverages the unique luminescent properties of lanthanides which include multiple, narrow emission bands and ms-scale excited state lifetimes. These features enable highly sensitive and multiplexed diagnostic assays, high throughput screening methods or optical imaging approaches that employ time-gated detection strategies.
Body
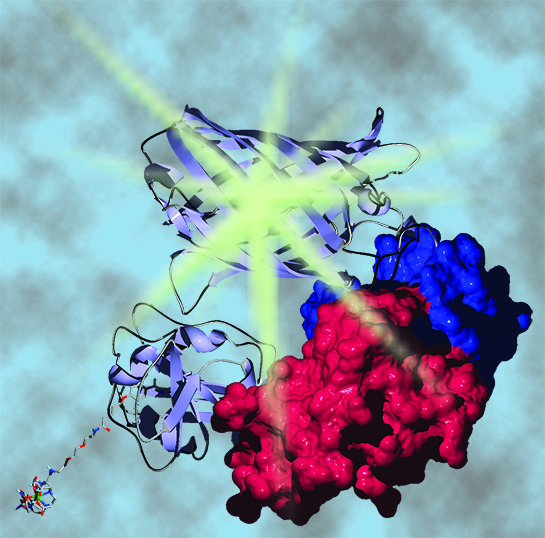
Lanthanide-based Förster resonance energy transfer (FRET) biosensors for time-gated imaging. FRET is non-radiative energy transfer from a donor fluorophore to a nearby (<10 nm) acceptor fluorophore, and FRET microscopy is widely used to dynamically visualize protein-protein interactions in live cells. Tb(III) and Eu(III) chromophores are excellent donors for multiplexed FRET applications because they can sensitize emission of multiple, differently colored acceptors with single-wavelength excitation. With time-gating, a brief (1 –10 µs) delay is inserted between pulsed, near-UV excitation and detection in order to separate long-lived lanthanide emission from ns-scale sample autofluorescence and directly excited acceptor fluorescence, yielding high signal-to-background ratio images. Our lab has pioneered lanthanide-based FRET imaging in live cells (PNAS, 2010). We’ve developed cell permeable Tb(III) protein labels (Chemistry, 2012; Bioconjugate Chemistry, 2015), built and evaluated time-gated microscopes (Biophysical Journal, 2015), and demonstrated live-cell FRET imaging that is 100-fold more sensitive than conventional FRET with fluorescent proteins (Science Advances, 2016). We continue to optimize the lanthanide-based approach to FRET microscopy and through collaborations are applying our methods to studies of protein complex dynamics.
Body
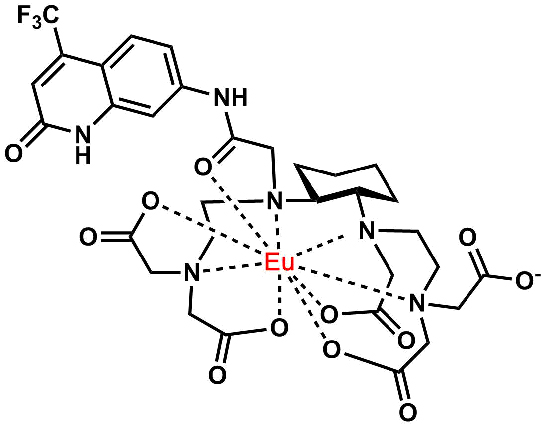
Metal complex probe development. Tb(III) and Eu(III) luminophores, Gd(III) magnetic resonance contrast agents, radiopharmaceuticals and other metallobioprobes must meet a number of practical requirements including highly stable chelation, efficient synthetic routes to preparation and easy modification for bioconjugation. We are actively developing functional metal complexes including brightly emissive and stable Tb(III) and Eu(III) species based on a cyclohexyl, triethylene tetraamine hexaacetic acid (cyTTHA) chelator scaffold (Bioconjugate Chemistry, 2016).
High-throughput screening (HTS) assays. Time-gated detection with lanthanide probes is a mainstay of drug discovery efforts. We have developed probes and assays for HTS (Chembiochem, 2012; Chemistry, 2016) and are actively engaged in this field.
.
.
Selected Publications
1. Yao, C. Kong, L. Yin, A.D. Jain, K. Ratia, G.R. Thatcher, T.W. Moore, T.G. Driver, L.W. Miller Time-Gated Detection of Cystathionine γ-Lyase Activity and Inhibition with a Selective, Luminogenic Hydrogen Sulfide Sensor. 2017 Chem. – Eur. J. 23, 752-756.
2. Mohamadi, L.W. Miller Brightly Luminescent and Kinetically Inert Lanthanide Bioprobes Based on Linear and Preorganized Chelators. 2016 Bioconjug. Chem. 27, 2540-2548.
3. S. Afsari, M. Cardoso Dos Santos, S. Lindén, T. Chen, X. Qiu, P. M. P. van Bergen en Henegouwen, T.L. Jennings , S. Kimihiro, I.L. Medintz, N. Hildebrandt, L.W. Miller. Time-gated FRET nanoassemblies for rapid and sensitive intra- and extracellular fluorescence imaging. 2016 Sci. Adv. 2, e1600265.
4. Rajendran, L.W. Miller. Evaluating the Performance of Time-Gated Live-Cell Microscopy with Lanthanide Probes. 2015 Biophys. J. 109, 240-248.
5. Zou, M. Rajendran, D. Magda, L.W. Miller. Cytoplasmic delivery and selective, multicomponent labeling with oligoarginine-linked protein tags. 2015 Bioconjug. Chem. 26, 460-465.
6. Rajendran, E. Yapici, L.W. Miller. Lanthanide-based imaging of protein-protein interactions in live cells. 2014 Inorg. Chem. 53, 1839-1853.
7. Mohandessi, M. Rajendran, D. Magda, L.W. Miller. Cell-Penetrating Peptides as Delivery Vehicles for a Protein-Targeted Terbium Complex. 2012 Chem. Eur. J. 18, 10825-10829.
8. Yapici, D.R. Reddy, L.W. Miller. An Adaptable Luminescence Resonance Energy Transfer Assay for Measuring and Screening Protein–Protein Interactions and their Inhibition. 2012 Chembiochem 13, 553-558.
9. E. Rajapakse, N. Gahlaut S. Mohandessi, D. Yu D, J.R. Turner, L.W. Miller. Time-resolved luminescence resonance energy transfer imaging of protein–protein interactions in living cells. 2010 Proc. Natl. Acad. Sci. USA 107:13582-13587.
Education
B.S. 1989, The University of Wisconsin-Madison
Ph.D. 1998, The University of Wisconsin-Madison
Postdoctoral/Associate Research Scientist 2001-2006, Columbia University