Luke Hanley
LAS Distinguished Professor
Contact
Address:
5417-1 (red door) SES, MC 111
Office Phone:
Email:
Related Sites:
About Heading link
Photoionization is the event in which an atom, molecule, cluster, or solid is illuminated with light and thereby ejects an electron, leaving behind a positive charge. Photoionization is the initiating event for most of the various experimental methods central to my research: ultrashort pulse laser ablation of solid material, photoionization of gas phase molecules, and X-ray photoelectron spectroscopy of surfaces. Our research applies photoionization to surface chemical analysis to address problems in analytical chemistry, mass spectrometry, microbiology, bioengineering, and surface science. This work often requires us to construct novel instrumentation in our own laboratory, but we also use advanced instrumentation located in UIC’s centralized instrumentation facility (the Research Resources Center) or synchrotron radiation research user facilities.
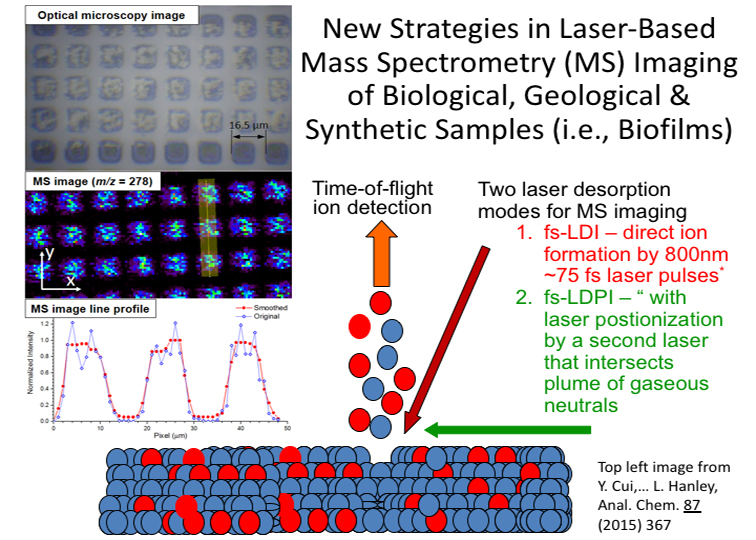
- Laser Desorption Postionization Mass Spectrometric Imaging of Biofilms & Organic Films. Mass spectrometric imaging is used to probe bacterial biofilms, mammalian tissues, and other complex organic films with micron lateral and depth resolution. Molecular and elemental species are detected within intact samples in a method we have advanced called laser desorption postionization mass spectrometry (LDPI-MS) imaging. We mostly use LDPI-MS imaging and ancillary methods to probe metabolites, antibiotics, proteins, and peptides within intact biofilms with the ultimate goal of advancing novel methods of biofilm control.
- Applying Methods of Advanced Surface Analysis. We also use X-ray photoelectron spectroscopy and related methods to probe elemental and functional group composition of surfaces. This work has also led us to the use of ultraviolet photoelectron spectroscopy, near edge X-ray absorption spectroscopy, X-ray surface scattering, transmission and scanning electron microscopy, linear and nonlinear optical absorption of films, quartz crystal microbalance measurement of film deposition or removal, X-ray diffraction, atomic force microscopy, and profilometry.
Selected Publications
Since 2011 only, out of 131 total refereed & 15 non-refereed. Senior author is underlined.
134. “Laser desorption combined with laser postionization for mass spectrometry”, L. Hanley, R. Wickramasinghe, and Y.P. Yung, Annu. Rev. Anal. Chem. 12 (2019) invited review, now online: https://doi.org/10.1146/annurev-anchem-061318-115447
133. “Femtosecond laser desorption ionization mass spectrometry imaging and multivariate analysis of lipids in pancreatic tissue”, A.V. Walker, L.D. Gelb, G.E. Barry, P. Subanajouy, A. Poudel, M. Hara, I.V. Veryovkin, G.I. Bell, and L. Hanley, Biointerphases 13 (2018) 03B416. http://doi.org/10.1116/1.5016301
132. “Competitive resource allocation to metabolic pathways contributes to overflow metabolisms and emergent properties in cross feeding consortia”, R.P. Carlson, A.E. Beck, P. Phalak, M.W. Fields, T. Gedeon, L. Hanley, W.R. Harcombe, M.A. Henson, and J.J. Heys, Biochem. Soc. Trans. 46 (2018) 269-284. http://doi.org/10.1042/bst20170242
131. “Solid sampling with a diode laser for portable ambient mass spectrometry”, Y.P. Yung, R. Wickramasinghe, A. Vaikkinen, T.J. Kauppila, I.V. Veryovkin, and L. Hanley, Anal. Chem. 89 (2017) 7297-7301. http://dx.doi.org/10.1021/acs.analchem.7b01745, PMCID: PMC5518277
130. “Metal impurity-assisted formation of nanocone arrays on Si by low energy ion-beam irradiation”, K. Steeves Lloyd, I.L. Bolotin, M. Schmeling, L. Hanley, I.V. Veryovkin, Surf. Sci. 652 (2016) 334-343. http://dx.doi.org/10.1016/j.susc.2016.03.016
129. “ChiMS: Open-source instrument control software platform on LabVIEW for imaging/depth profiling mass spectrometers”, Y. Cui and L. Hanley, Rev. Sci. Instrum. 86 (2015) 065106. http://dx.doi.org/10.1063/1.4922913 PMCID: 4482810
128. “High lateral resolution vs. molecular preservation in near-IR fs-laser desorption postionization mass spectrometry”, Y. Cui, I.V. Veryovkin, M.W. Majeski, D.R. Cavazos, and L. Hanley, Anal. Chem. 87 (2015) 367-371. http://dx.doi.org/10.1021/ac5041154 (open access)
127. “Internal energy of thermometer ions formed by femtosecond laser desorption: Implications for mass spectrometric imaging”, S. Milasinovic, Y. Cui, R.J. Gordon and L. Hanley, J. Phys. Chem. C 118 (2014) 28938-28947. http://dx.doi.org/10.1021/jp504062u (open access)
126. “Cluster beam deposition of Cu2-XS nanoparticles into organic thin films”, M.W. Majeski, I.L. Bolotin, and L. Hanley, ACS Appl. Mater. Interf. 6 (2014) 12901-12908. http://dx.doi.org/10.1021/am5028428
125. “Ion sources for mass spectrometric identification and imaging of molecular species”, C. Bhardwaj and L. Hanley, Nat. Prod. Rep. 31 (2014) 756-767. http://dx.doi.org/10.1039/C3NP70094A
Notable Honors
1994-1999, National Science Foundation Young Invest, National Science Foundation
1995-1998, UIC Junior Scholar, UIC
1997, Teaching Recognition Award, UIC
Education
University of Toronto, 1983
PhD, State University of New York at Stony Brook, 1988
NSF Postdoctoral Fellow, University of Pittsburgh, 1988-1990